 PDF(7995 KB)
PDF(7995 KB)


屋尘螨变应原Der p 33重组蛋白制备及血清IgE结合率
杨李, 王楠, 滕飞翔, 马桂芳, 孙金霞
中国媒介生物学及控制杂志 ›› 2023, Vol. 34 ›› Issue (1) : 21-25.
 PDF(7995 KB)
PDF(7995 KB)
 PDF(7995 KB)
PDF(7995 KB)
屋尘螨变应原Der p 33重组蛋白制备及血清IgE结合率
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Preparation of the recombinant protein of Der p 33 from Dermatophagoides pteronyssinus and determination of its serum IgE-binding rate
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}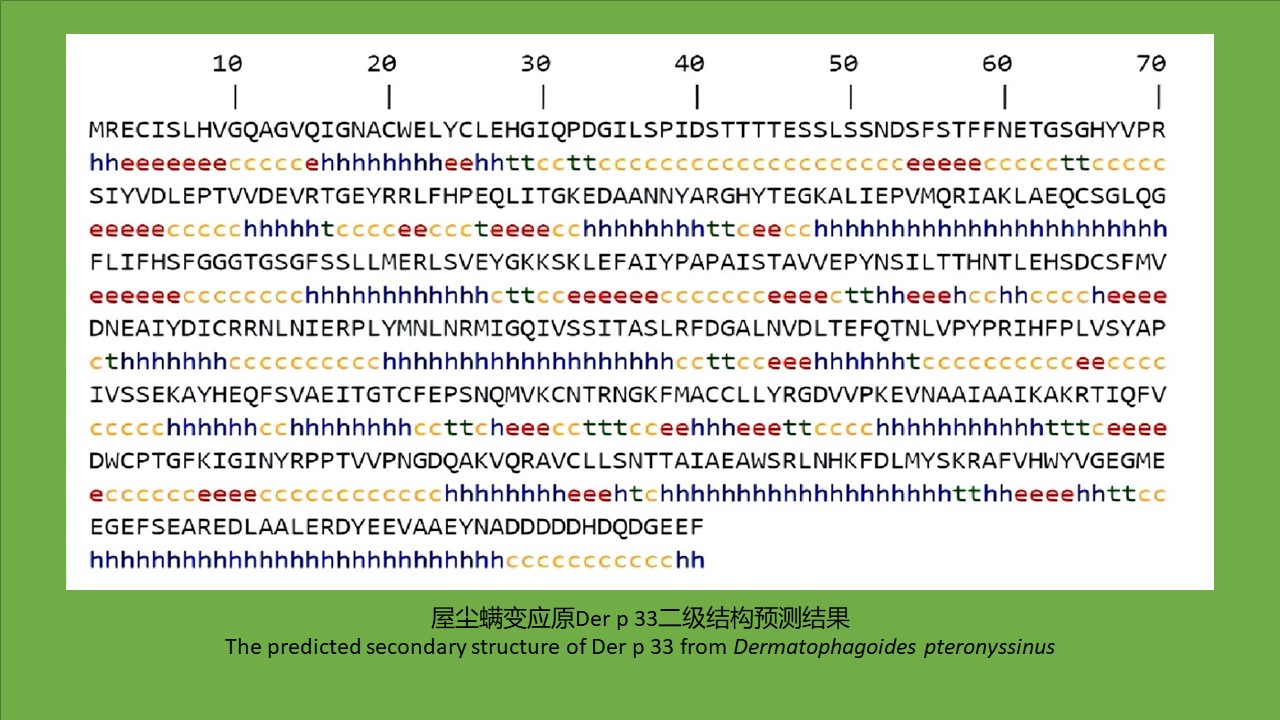
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |