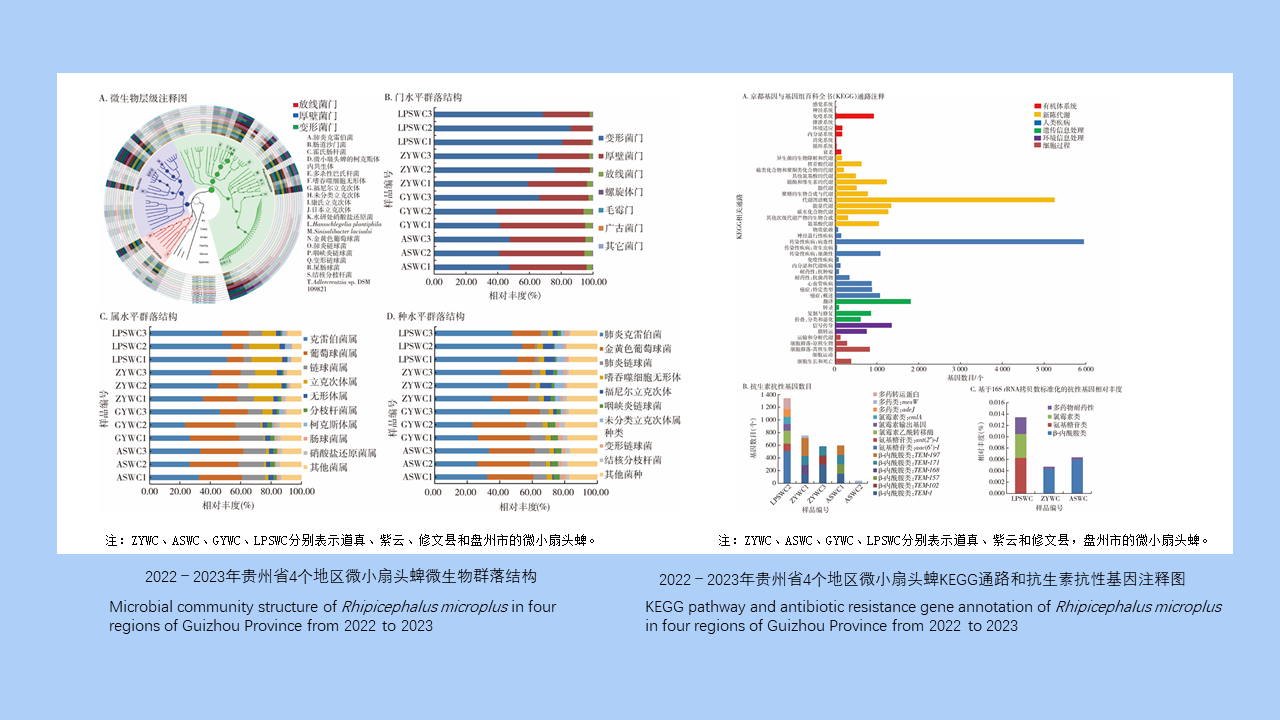
Objective To understand the microbial community and antibiotic resistance genes (ARGs) in Rhipicephalus microplus in Guizhou Province, China. Methods From April to May in 2022 and 2023, R. microplus was collected from cattle surface in Daozhen, Ziyun, Xiuwen counties, and Panzhou City of Guizhou Province using the method of examining ticks on animal body surface. The collected tick specimens were grouped by region, and 15 female ticks were randomly selected from each region and divided into 3 tubes for metagenomic sequencing. The sequencing data were subjected to quality control, splicing, and assembly, followed by comparison against the structured antibiotic resistance gene database and the non-redundant protein library of the National Center for Biotechnology Information to obtain the annotation information of species and ARGs from each specimen. Then visual analysis was performed using R 3.6.3 and GraPhlAn 1.1.3, including taxonomical composition, ARG composition, and group similarities. Results A total of 550 blood-sucking R. microplus were collected, including 404 females, 85 males, and 61 nymphs. The dominant phylum of bacteria in R. microplus was Proteobacteria, with an average relative abundance of 60.01%, followed by Firmicutes (36.76%). The dominant genus was Klebsiella (38.58%), followed by Staphylococcus (22.00%). The dominant species was K. pneumoniae (38.58%), followed by Sta. aureus (22.00%). In addition, tick-borne pathogens such as Anaplasma phagocytophilum, Rickettsia fournieri, R. conorii, R. japonica, Borrelia garinii, and Coxiella burnetii were also detected, accounting for 3.08%, 3.04%, 0.76%, 0.70%, 0.01%, and 0.01%, respectively. The analysis of group similarities showed that the difference between groups was greater than that within groups (R=0.586, P=0.002). ARG annotation identified 3 316 genes related to resistance against β-lactams (54.22%), aminoglycosides (20.18%), chloramphenicols (13.61%), and multidrugs (11.99%). The types and abundance of ARGs were different in different regions. Conclusions The microflora in R. microplus in Guizhou Province is diverse, and there are many types of pathogens in R. microplus. Moreover, R. microplus carries a large number of ARGs. Therefore, it is necessary to strengthen the study of tick microflora and resistance genes, so as to guide the rational use of drugs and prevent the occurrence of tick-borne diseases.
[1] Cao GP, Zhan BD, Zhong JY,et al. Status of tick distribution and tick-borne pathogens in urban parks of Quzhou,Zhejiang,2017-2019[J]. Dis Surveill,2021,36(9):879-883. DOI:10.3784/jbjc.202106010314.(in Chinese)曹国平,占炳东,钟建跃,等. 2017—2019年浙江省衢州市城区公园蜱分布及携带病原体情况调查[J]. 疾病监测,2021,36(9):879-883. DOI:10.3784/jbjc.202106010314.
[2] Beard D, Stannard HJ, Old JM. Parasites of wombats (family Vombatidae),with a focus on ticks and tick-borne pathogens[J]. Parasitol Res,2021,120(2):395-409. DOI:10.1007/s00436-020-07036-0.
[3] Barker SC, Walker AR. Ticks of Australia. The species that infest domestic animals and humans[J]. Zootaxa,2014,3816(1):1-144. DOI:10.11646/zootaxa.3816.1.1.
[4] Zhao GP, Wang YX, Fan ZW,et al. Mapping ticks and tick-borne pathogens in China[J]. Nat Commun,2021,12(1):1075. DOI:10.1038/s41467-021-21375-1.
[5] Xiang YL, Zhou JZ, Yu FX,et al. Characterization of bacterial communities in ticks parasitizing cattle in a touristic location in southwestern China[J]. Exp Appl Acarol,2023,90(1/2):119-135. DOI:10.1007/s10493-023-00799-y.
[6] Thanchomnang T, Rodpai R, Thinnabut K,et al. Characterization of the bacterial microbiota of cattle ticks in northeastern Thailand through 16S rRNA amplicon sequencing[J]. Infect Genet Evol,2023,115:105511. DOI:10.1016/j.meegid.2023.105511.
[7] Lu M, Tang GP, Ren ZQ,et al. Ehrlichia,Coxiella and Bartonella infections in rodents from Guizhou Province,southwest China[J]. Ticks Tick Borne Dis,2022,13(5):101974. DOI:10.1016/j.ttbdis.2022.101974.
[8] Jia N, Wang JF, Shi WQ,et al. Large-scale comparative analyses of tick genomes elucidate their genetic diversity and vector capacities[J]. Cell,2020,182(5):1328-1340.e13. DOI:10.1016/j.cell.2020.07.023.
[9] Diop A, Barker SC, Eberhard M,et al. Rickettsia fournieri sp. nov.,a novel spotted fever group Rickettsia from Argas lagenoplastis ticks in Australia[J]. Int J Syst Evol Microbiol,2018,68(12):3781-3784. DOI:10.1099/ijsem.0.003057.
[10] Xiang YL, Zhou JZ, Zhang Y,et al. Metagenomic analysis of Rhipicephalus microplus from minority autonomous prefectures in Guizhou Province,China[J]. Chin J Vector Biol Control,2023,34(3):319-325. DOI:10.11853/j.issn.1003.8280.2023.03.007.(in Chinese)向昱龙,周敬祝,张燕,等. 贵州省少数民族自治州微小扇头蜱的宏基因组分析[J]. 中国媒介生物学及控制杂志,2023,34(3):319-325. DOI:10.11853/j.issn.1003.8280.2023.03.007.
[11] Han J, He Z, Shao ZJ. The research progress of common tick-borne Rickettsia[J]. Chin J Hyg Insect Equip,2022,28(1):86-89. DOI:10.19821/j.1671-2781.2022.01.024.(in Chinese)韩婧,贺真,邵中军. 常见蜱传立克次体的研究进展[J]. 中华卫生杀虫药械,2022,28(1):86-89. DOI:10.19821/j.1671-2781.2022.01.024.
[12] Spernovasilis N, Markaki I, Papadakis M,et al. Mediterranean spotted fever: Current knowledge and recent advances[J]. Trop Med Infect Dis,2021,6(4):172. DOI:10.3390/tropicalmed6040172.
[13] Li JB, Hu W, Wu T,et al. Japanese spotted fever in eastern China,2013[J]. Emerg Infect Dis,2018,24(11):2107-2109. DOI:10.3201/eid2411.170264.
[14] Qin XR, Han HJ, Han FJ,et al. Rickettsia japonica and novel Rickettsia species in ticks,China[J]. Emerg Infect Dis,2019,25(5):992-995. DOI:10.3201/eid2505.171745.
[15] Wang Q, Guo WB, Pan YS,et al. Detection of novel spotted fever group rickettsiae (Rickettsiales:Rickettsiaceae) in ticks (Acari:Ixodidae) in southwestern China[J]. J Med Entomol,2021,58(3):1363-1369. DOI:10.1093/jme/tjaa294.
[16] Lu M, Meng C, Zhang B,et al. Prevalence of spotted fever group Rickettsia and Candidatus Lariskella in multiple tick species from Guizhou province,China[J]. Biomolecules,2022,12(11):1701. DOI:10.3390/biom12111701.
[17] Lu M, Meng C, Gao X,et al. Diversity of Rickettsiales in Rhipicephalus microplus ticks collected in domestic ruminants in Guizhou Province,China[J]. Pathogens,2022,11(10):1108. DOI:10.3390/pathogens11101108.
[18] Bao L, Li YY, Li HY,et al. Epidemiological characteristics and influencing factors of typhus in Xishuangbanna Dai Autonomous Prefecture,Yunnan Province,China,2016-2020[J]. Chin J Vector Biol Control,2022,33(6):854-858. DOI:10.11853/j.issn.1003.8280.2022.06.017.(in Chinese)包蕾,李园园,李海艳,等. 云南省西双版纳州2016—2020年斑疹伤寒流行特征及影响因素研究[J]. 中国媒介生物学及控制杂志,2022,33(6):854-858. DOI:10.11853/j.issn. 1003. 8280.2022.06.017.
[19] Wu XB, Na RH, Wei SS,et al. Distribution of tick-borne diseases in China[J]. Parasit Vectors,2013,6(1):119. DOI:10.1186/1756-3305-6-119.
[20] Estrada-Peña A, De La Fuente J. The ecology of ticks and epidemiology of tick-borne viral diseases[J]. Antiviral Res,2014,108:104-128. DOI:10.1016/j.antiviral.2014.05.016.
[21] Li SS, Zhang XY, Zhou XJ,et al. Bacterial microbiota analysis demonstrates that ticks can acquire bacteria from habitat and host blood meal[J]. Exp Appl Acarol,2022,87(1):81-95. DOI:10.1007/s10493-022-00714-x.
[22] Xue XL, Han AX, Ye SQ, et al. Metagenomic analysis of microbial community structure, antibiotic resistance genes, and virulence factors of ticks captured at Wenzhou port, Zhejiang Province, China[J]. Chin J Vector Biol Control, 2021, 32(6):763-771. DOI:10.11853/j.issn.1003.8280.2021.06.021.(in Chinese)许雪莲,韩阿祥,叶诗晴,等.温州口岸截获蜱体内微生物群落结构、抗生素抗性基因及毒力因子的宏基因组分析[J].中国媒介生物学及控制杂志,2021,32(6):763-771. DOI:10.11853/j.issn.1003.8280.2021.06.021.
[23] Wei NN, Lu JM, Dong Y,et al. Profiles of microbial community and antibiotic resistome in wild tick species[J]. mSystems,2022,7(4):e0003722. DOI:10.1128/msystems.00037-22.
[24] Chavarría-Bencomo IV, Nevárez-Moorillón GV, Espino-Solís GP,et al. Antibiotic resistance in tick-borne bacteria:A one health approach perspective[J]. J Infect Public Health,2023,16Suppl 1:153-162. DOI:10.1016/j.jiph.2023.10.027.
[25] Papp M, Tóth AG, Valcz G,et al. Antimicrobial resistance gene lack in tick-borne pathogenic bacteria[J]. Sci Rep,2023,13(1):8167. DOI:10.1038/s41598-023-35356-5.
[26] Zhang WM, Yu CX, Yin SQ,et al. Transmission and retention of antibiotic resistance genes (ARGs) in chicken and sheep manure composting[J]. Bioresour Technol,2023,382:129190. DOI:10.1016/j.biortech.2023.129190.


